1、When preparing for testing, open the aluminum foil bag from the tear opening. Take out the test card and lay it flat on the horizontal desktop.
2.Disinfect the testers fingertips with an alcohol casas reps gently pinch the fingertip once using the lancets as weeds out the block
3. Take 10ul of serum and plasma samples to be tested or 15ul of whole blood samples from the sample tube with the sample gun and add them into the sample hole on the test card.
Immediately add 2 drops (about 80-100ul) of diluent into the sample hole, and ensure that there is no bubble during the operation.
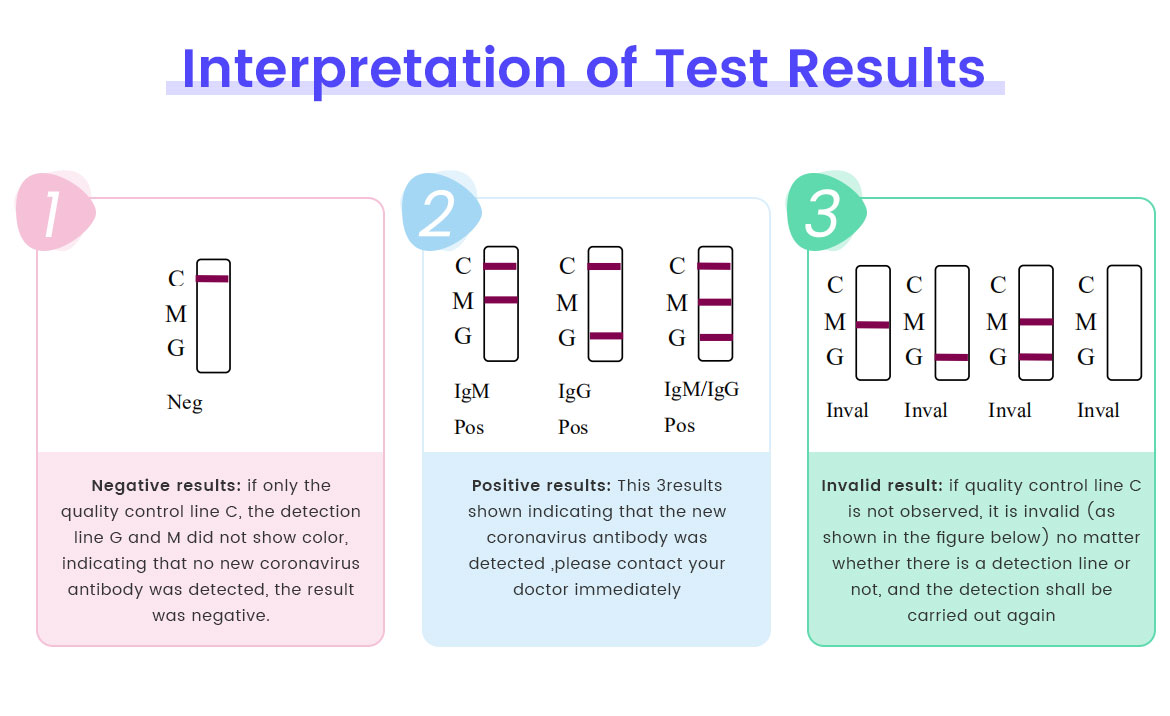
4. Time and interpret the results within 15 minutes.
5. Do not read the result after 15 minutes. After observing and recording the results, please discard the detection card to avoid confusing the result judgment. For long-term preservation, please take photos of the results.
【Intended Use】
This kit is used for the qualitative detection of 2019-nCoV IgG antibody in human serum, plasma or whole blood in vitro.
Coronavirus belongs the genus Coronavirus of the family Coronaviridaein systematic classification. It is enveloped positive-sense single-stranded RNA virus, with a diameter of about 80-120nm. Its genetic material is the largest among all RNA viruses, and it only infects human, rats, pigs, cats, dogs and bird vertebrates. A variant of Coronavirus is the pathogen that causes SARS and belongs to RNA viruses.
When the human body comes in contact with foreign antigens, the earliest antibody produced is IgM, which is directly secreted by B cell receptor. B cellsproducing IgM enter the lymph nodes and are stimulated by T cells and antigenpresenting cells at the generative center. Then, they grown and differentiate into plasma cells and producea large amount of IgG.
【Test Principle】
The novel coronavirus novel coronavirus antigen and quality control antibody were detected by colloidal gold immunochromatography, and the detection card contained
1. Colloidal gold labeled new type of coronavirus antigen and quality control antibody;
2. There were two lines (G line and M line) and a cellulose nitrate membrane on the quality control line (C line). The G line was fixed with anti human IgG antibody, which was used to detect the new coronavirus IgG antibody. M line was fixed withanti human IgM antibody, which was used to detect the new corona. IgM antibody of virus; quality control antibody was fixed on line C
【Storage Conditions and Validity】
1. Storage conditions: store at 4-30 ℃.
2. Product validity: the products that are not opened shall be stored at 4-30 ℃ for 12 months; the inspection card will be invalid due to moisture absorption after opening the inner packaging, please use it within 1 hour.
【Sample Requirements】
1. It is applicable to human serum, plasma or whole blood samples, including plasma or whole blood samples prepared by clinical commonly used anticoagulants (EDTA, sodium citrate).
2. After the sample is collected, it shall be tested immediately. If it cannot be tested immediately, the serum and plasma samples to be tested can be stored at 2-8 ℃ for 5 days. If long-term storage is needed, it should be placed at - 20 ℃ to avoid repeated freezing and thawing; the whole blood sample of anti doubt should not be stored for more than 72 hours at normal temperature, and for less than 7 days at 2-8 ℃.
3. Before testing, slowly restore the refrigerated or frozen samples to room temperature and mix them carefully. When
there is a visible particulate matter in the sample, it should be centrifuged before the test to remove the precipitate.
4. If there is a lot of lipid, hemolysis or turbidity in the sample, please do not use it to avoid affecting the result judgment.
【Limitations of test methods】
1. This product is only used for qualitative test in vitro.
2. The test results of this product are not the only basis for diagnosis, and should be combined with clinical symptoms or other routine examination methods for diagnosis.
3. Limited by the detection sensitivity, the negative result may be due to the antibody concentration lower than the yield It is caused by the sensitivity of product analysis
【Attention】
1. This product is only used for external diagnosis. The operation shall be carried out in strict accordance with the operation manual. Do not use expired or damaged products.
2. Only the matching diluent in the package can be used, and the diluent of different batches cannot be mixed use
3. Do not use tap water, purified water and distilled water as negative control.
4. The test card shall be used within 1 hour after unsealing; if the ambient temperature is higher than 30 ℃ or wet, it shall be used immediately after opening.
5. If there is no liquid moving in the test window within 30 seconds after dropping the test liquid, then Add 1 drop of detection solution.
6. When collecting samples, pay attention to the possibility of being infected by the virus and take disposable hands
Cover, mask, etc. then wash hands.
7. This test card is disposable. The test card and sample after use shall be regarded as Medical wastes with risk of biological infection shall be disposed properly in accordance with relevant national regulations
【Reference】
Regulations on the management of instructions and labels of medical devices (Order No. 6 of the State Food and Drug
Administration) Guidelines for preparation of instructions for in vitro diagnostic reagents (No. 17, 2014)

