XIII. Drug Use Management of COVID-19 Patients
COVID-19 patients are often complicated with underlying diseases receiving multiple types of
drugs. Therefore, we should pay more attention to the adverse drug reactions and drug
interactions so as to avoid drug-induced organ damage and improve the success rate of
treatment.
1、Identification of adverse drug reactions
It has been demonstrated that the incidence of abnormal liver function is 51.9% in
COVID-19 patients who have received lopinavir/ritonavir combined arbidol antiviral
treatment. Multivariate analysis revealed that antiviral agents and more concomitant
medications are two independent risk factors of abnormal liver function. Therefore,
monitoring of the adverse drug reactions should be strengthened; the unnecessary
drug combinations should be reduced. The main adverse reactions of antiviral agents
include:
{l) Lopinavir /ritonavir and darunavir/cobicistat: diarrhea, nausea, vomit, the increase
of serum aminotransferase, jaundice, dyslipidemia, the increase of lactic acid.
Symptoms will recover after drug withdrawal.
{2) Arb idol: the increase of serum aminotransferase and jaundice. When combined with
lopinavir, the incidence rate is even higher. The symptoms will recover after drug
withdrawal. Sometimes a slowdown of the heart could be induced; thus it is necessary
to avoid the combination of arbidol with ~-receptor inhibitors such as metoprolol and
propranolol. We suggest to stop taking the drugs when the heart rate drops below
60/min.
{3) Fapilavir: elevation of plasma uric acid, diarrhea, neutropenia, shock, fulminant
hepatitis, acute kidney injury. The adverse reactions were commonly seen in elderly
patients or patients complicated with cytokine storm.
(4) Chloroquine phosphate: dizziness, headache, nausea, vomit, diarrhea, different
kinds of skin rash. The most severe adverse reaction is cardiac arrest. The main adverse
reaction is the ocular toxicity. An electrocardiogram needs to be examined before
taking the drug. The drug should be prohibited for patients with arrhythmia (e.g.,
conduction block), retinal disease, or hearing loss.
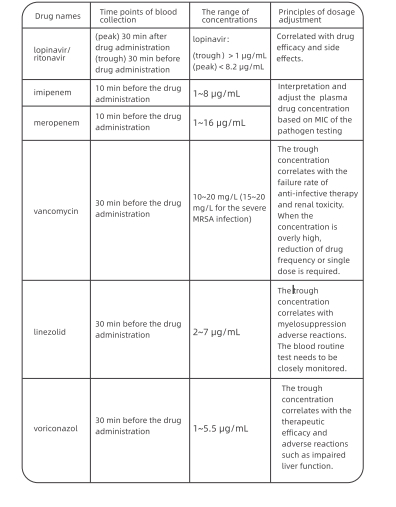
2 Therapeutic Drug Monitoring
Some antiviral and antibacterial drugs need therapeutic drug monitoring {TDM). Table
1 presents the plasma concentrations of such drugs and their dosage adjustment. Upon
the onset of aberrations of plasma drug concentration, the treatment regimens need to
be adjusted by considering the clinical symptoms and concomitant drugs.
Table 1 The range of concentrations and points for attention of the common
TDM drugs forthe C0VID-19 patients
Paying attention to the potential drug interactions
Antiviral drugs such as lopinavir/ritonavir are metabolized through the enzyme CYP3A
in the liver. When patients receiving concomitant medications, the potential drug
interactions need to be carefully screened. Table 2 shows interactions between antiviral
drugs and common drugs for underlying diseases.
Table 2 Interactions between antiviral drugs and common drugs for underlying
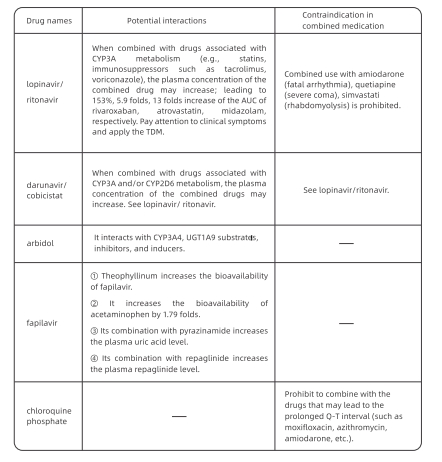
Note: "-" : no relevant data; TDM: therapeutic drug monitoring; ALIC: area under the curve;
LIGT1A9: uridine diphosphate glucosidase 1A9.
Avoiding medical damage in special populations
Special populations include pregnant women, patients with hepatic and renal
insufficiency, patients supported by mechanical ventilation, patients under continuous
renal replacement therapy {CRRT) or, extra corporeal membrane oxygenation {ECMO), etc.
The following aspects need to be noted during drug administration.
{1) Pregnant women
Lopinavir/ritonavir tablets could be used. Favipiravir and chloroquine phosphate are
prohibited.
{2) Patients with hepatic insufficiency Drugs that are excreted unchanged through the
kidney are preferred, such as penicillin and cephalosporins, etc.
(3) Patients with renal insufficiency (including those on hemodialysis)
Drugs that are metabolized through the liver or excreted through the liver-kidney double
channels are preferred, such as linezolid, moxifloxacin, ceftriaxone, etc.
(4) Patients under CRRT for 24h Forvancomycin, the recommended regimen is: loading dose 1 g
and maintenance dose 0.5 g, ql 2h. For imipenem, the maximum daily dosage should not exceed
2g




